There are two main sources of sodium carbonate:
a) from salt and calcium carbonate (via the ammonia soda (Solvay) process)
b) from sodium carbonate and hydrogencarbonate ores (trona and nahcolite)
(a) From sodium chloride and calcium carbonate
The overall reaction can be regarded as between calcium carbonate and sodium chloride:

However, calcium carbonate is too insoluble to react with a solution of salt. Instead the product is obtained by a series of seven stages.
The process is known as the ammonia-soda process or the Solvay process, named after the Belgian industrial chemist who patented it in 186I.
The various stages of the Solvay process are interlinked as can be seen from the diagram and description below.

(1) Ammoniation of brine
Ammonia gas is absorbed in concentrated brine to give a solution containing both sodium chloride and ammonia. Na+(aq), Cl–(aq), NH4+(aq), OH–(aq) ions and NH3(aq) are present.
(2) Formation of calcium oxide and carbon dioxide
Kilns are fed with a limestone/coke mixture (13:1 by mass). The coke burns in a counter-current of pre-heated air:
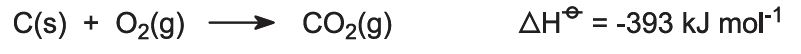
The heat of combustion raises the temperature of the kiln and the limestone decomposes:
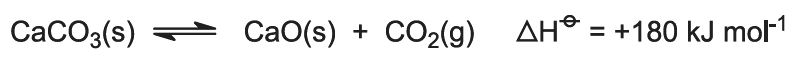
The gas, containing approximately 40% carbon dioxide, is freed of lime dust and sent to the carbonating (Solvay) towers. The residue, calcium oxide, is used in ammonia recovery (see step 7 below).
(3) The Solvay Tower
This is the key stage in the process. The ammoniated brine from step (1) is passed down through the Solvay Tower while carbon dioxide from steps (2) and (5) is passed up it. The Solvay Tower is tall and contains a set of mushroom-shaped baffles to slow down and break up the liquid flow so that the carbon dioxide can be efficiently absorbed by the solution. Carbon dioxide, on dissolving, reacts with the dissolved ammonia to form ammonium hydrogencarbonate:
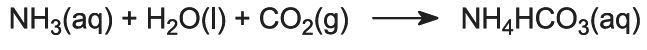
The solution now contains ions Na+(aq), Cl–(aq), NH4+(aq) and HCO3–(aq). Of the four substances which could be formed by different combinations of these ions, sodium hydrogencarbonate (NaHCO3) is the least soluble. It precipitates as a solid in the lower part of the tower, which is cooled. The net process is:
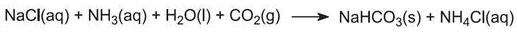
A suspension of solid sodium hydrogencarbonate in a solution of ammonium chloride is run out of the base of the tower.
(4) Separation of solid sodium hydrocarbonate
The suspension is filtered to separate the solid sodium hydrogencarbonate from the ammonium chloride solution, which is then used in stage (7).
(5) Formation of sodium carbonate
The sodium hydrogencarbonate is heated in rotating ovens at 450 K so that it decomposes to sodium carbonate, water and carbon dioxide:
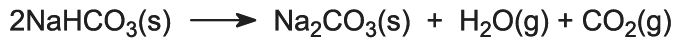
The carbon dioxide is sent back to the Solvay Tower for use in step (3). The product of the process, anhydrous sodium carbonate, is obtained as a fine white powder known as light sodium carbonate.
(6) Formation of calcium hydroxide
The last two stages, (6) and (7), are concerned with the regeneration of ammonia from ammonium chloride (made in step 3). The quicklime from step (2) is slaked with excess water giving milk of lime:

(7) Regeneration of ammonia
This calcium hydroxide suspension is mixed with the ammonium chloride solution left from step (4) and heated:

The ammonia is thus recovered, and sent back to step (1). Calcium chloride is the only by-product of the whole process.
The overall process is an elegant one. In theory, the only raw materials are limestone and brine. Inevitably, there are losses of ammonia, and these are made up for by addition of extra supplies, as required in step (1).